Please select your Region.
Please select your Region.
Jun 21, 2019
RSV is a common cause for respiratory infections during infancy. Typically, its symptoms are characterized by runny nose, fever, and cough, but respiratory distress is also observed in serious cases. Thus, there is a demand for fast diagnosis so that appropriate treatment may begin. Historically, RSV was prevalent in Japan during the winter season along with influenza, which causes similar respiratory infections. However, in more recent years RSV has begun to spread during summer.
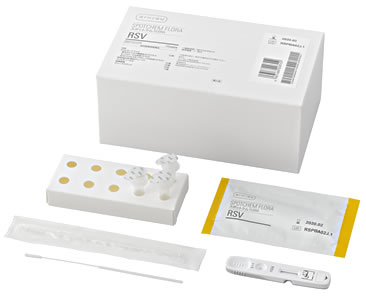
The "SPOTCHEM FLORA RSV", now sold by ARKRAY, Inc. (hereinunder, ARKRAY), is a kit for detecting RSV from fluid nasal swab samples. Early determination (fastest result: 1.5 minutes) is now possible due to high sensitivity measurement employing the TRF method* with the dedicated measurement instrument SPOTCHEM FLORA SF-5510/5520. By allowing for the rapid, high performance detection of viral infections, for which determination used to be difficult in the early stage, this kit will be beneficial to improving diagnosis efficiency, alleviating the burden on the patient and reducing opportunities for the spread of secondary infections.
By expanding test items, which already include the influenza virus and group A streptococcus antigen, ARKRAY will provide an even more versatile system to support the test-work of pediatricians and other medical facilities.
ARKRAY will continue to contribute to rapid diagnosis and appropriate treatment on the front line of the medical field through the development of new diagnosis systems.
*TRF method: Time-Resolved Fluorescence Method
○Simple, highly sensitive, automated measurement
By simply setting a cartridge containing sample into the instrument, the result is automatically determined with high sensitivity.
○Prevention against sample mix-ups
Names and ID numbers, etc., can be entered on the cartridges. Since this information is read in the instrument together with the inspection result, the cartridge is useful in preventing the mix-up of samples.
○Alleviating the burden of patients
In order to alleviate pain at the time of sample collection, soft-tipped flocked swabs are included.
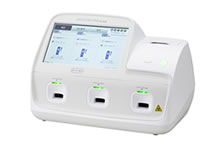
Portable Immunofluorometric Analyzer "SPOTCHEM FLORA SF-5510/SF-5520"
High detection performance is realized through the TRF method employing europium (Eu) as a labeling substance, which has a long fluorescence life and fluorescence wavelength that allows for easy detection of the virus. In the past, determining infection using conventional methods was difficult unless testing took place after the viral load increased in the body. However, it is now possible to determine infection even with a low viral load during the early stages of infection. Early determination can now be provided in as little as 1.5 minutes, and final determination in 10 minutes (influenza virus, RSV only). Depending on the model of instrument, 1 sample (SF-5510) to a maximum of 3 samples (SF-5520) can be measured.

SF-5510
SF-5520
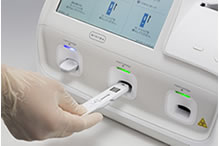
[Test procedure example]
(Test cartridge insertion)
| Name | RSV Kit "SPOTCHEM FLORA RSV" | |
|---|---|---|
| Release date | June 21, 2019 | |
| Specifications | ||
| Measurement subject | Nasal swab fluid | |
| Measurement item | RSV antigen | |
| Measurement device | Portable Immunofluorometric Analyzer SPOTCHEM FLORA SF-5510/SF-5520 | |
| Measurement principle | Fluorescence immunochromatographic method + TRF method (Time-Resolved Fluorescence method) | |
| Measurement time | 10 minutes per sample (early determination at 1.5 min, 2.5 min, 4 min and 6 min) | |
| Storage Method | 1 - 30°C, 12 months | |
| Packaging unit | 10 units (for 10 tests) (Test cartridges, sample extraction fluid, filter nozzles, tube stands, sterile swabs) | |
| Approval No | 23000EZX00060000 | |
| Product category | In-vitro Diagnostic Product | |
| Classification | Class III | |
The information presented in this release is limited to Japan as of the time of its announcement.
![]()