Please select your Region.
Please select your Region.
Jan 29, 2024
Launching the SARS-CoV-2/Flu (Types A/B)Diagnostic Kit
Helping Reduce Bottlenecking in Medical Settings by Shortening Test Times
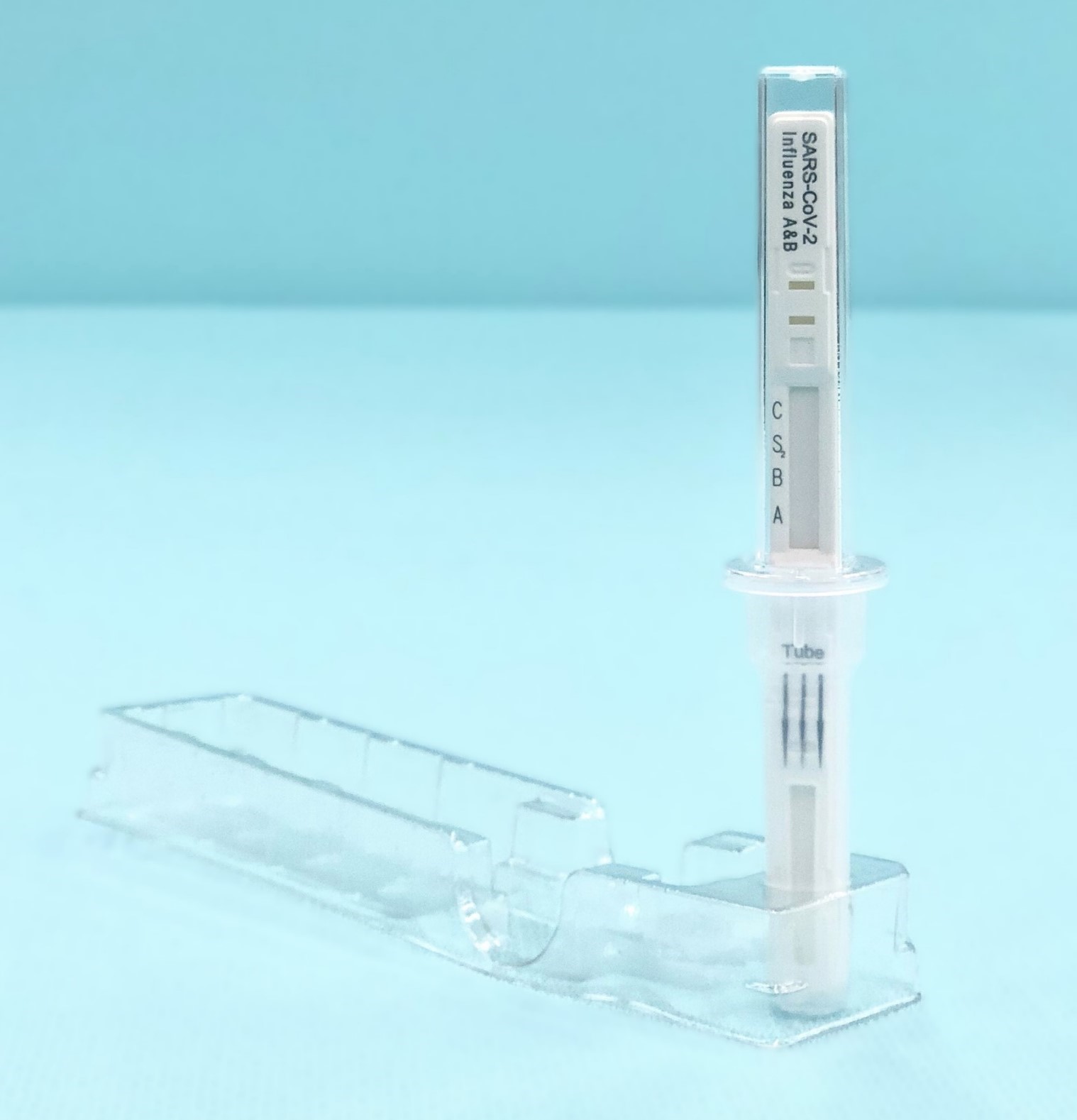
Primal Screen
This product has been designed by adopting the new form, "sealed dipping". It is an advanced form of the "dipping" and does not require the user to dispense drops of samples. The "dipping" type, which involves a test stick to be inserted into the extracted sample, does not require any dispensing of the sample,making testing simple and easy.On the other hand, it has the disadvantage of the sample being prone to adhering to fingers and other such surfaces, due to the exposed result window.
The "sealed dipping" model however, resolved this flaw by covering the result window with a case, while still keeping the benefits of the "dipping" format. In addition, by sealing the test stick with an attached tube cap, infection due to sample spillage or leakage is prevented.
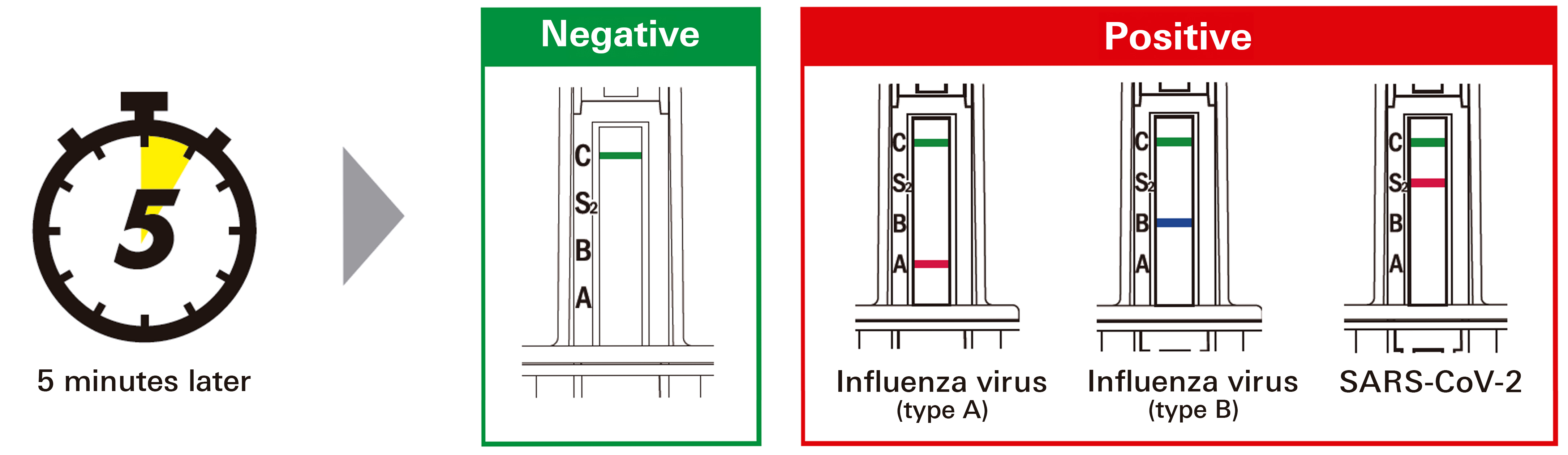
The test result is determined by the presence or absence of a line that appears in the result window of the test stick after it has been inserted into the sample and left to stand for five minutes. The multi-colored result window makes it easy to determine the results for the three types of antigens.
ARKRAY provides diagnostic tools for a variety of infectious diseases, including the novel coronavirus infections. ARKRAY will continue to rise to the needs of medical institutions by expanding our product lineup of diagnostic items for infectious diseases.
| Product name | Primal Screen SARS-CoV-2/Flu |
Product A | Product B | Product C | Product D |
|---|---|---|---|---|---|
| Time to results |
5 minutes | 15 minutes | 10 minutes | 15 minutes | 10 minutes |
| Application method |
Dipping | Two-point dispensing |
One-point dispensing |
One-point dispensing |
One-point dispensing |
| Name | Primal Screen |
|
|---|---|---|
| Intended use | Detection of SARS-CoV-2 antigens, influenza Type A virus antigens and influenza Type B virus antigens on nasopharyngeal or nasal swab (to assist in the diagnosis of SARS-CoV-2 infections or influenza virus infections) |
|
| Test object | Nasopharyngeal or nasal swab | |
| Time to results | 5 minutes (approx.) | |
| Testing principle | Immunochromatography | |
| Storage method | Room temperature (1 to 30°C) | |
| Shelf life | 12 months (expiration date is listed on the outer packaging) | |
| Product classification | In vitro diagnostics. Marketing Authorization No.: 30400EZX00011000 | |
| Sales price | JPY 29,500 (excluding tax) | |
This product will be distributed by ARKRAY Marketing, Inc. ARKRAY Marketing, Inc. is ARKRAY's distributor in Japan.
![]()